Number: Sa1615
SGLT2I REDUCES THE RISK OF DEVELOPING HCC IN PATIENTS WITH CO-EXISTING HEPATITIS C INFECTION AND TYPE 2 DIABETES: A GLOBAL MULTICENTER PROPENSITY-MATCHED STUDY
Society: AASLD
Track: Liver Diseases and Transplantation
Introduction:
Chronic Hepatitis C infection (CHC) and Diabetes Mellitus Type 2 (T2DM) are independent risk factors for the development of Hepatocellular Carcinoma (HCC). Preclinical studies suggest that Sodium-glucose cotransporter-2 receptor inhibitors (SGLT2i) regulate pathways implicated in HCC progression and may inhibit oncogenesis. However, clinical studies are lacking. Using a large research network, this study aimed to evaluate the effect of SGLT2i on the incidence of HCC in patients with concomitant T2DM and CHC.
Methods:
In this retrospective cohort study and time-to-event analysis, we utilized the TriNetX electronic health records network, encompassing over 120 million patients from January 1, 2005, to December 31, 2022. We included patients aged 18 years or older diagnosed with co-existing T2DM and patients with CHC (who had received either previous or current antiviral treatment). The patients with and without SGLT2i were matched by propensity score for age, gender, race, comorbidities, liver-related characteristics, BMI, and background medications. Patients with a prior history of HCC, liver transplant, diabetes mellitus type 1, and end-stage renal disease were excluded from the study. Using a Cox proportional hazards regression model, we evaluated the association between SGLT2i use and the incidence of HCC in both groups.
Results:
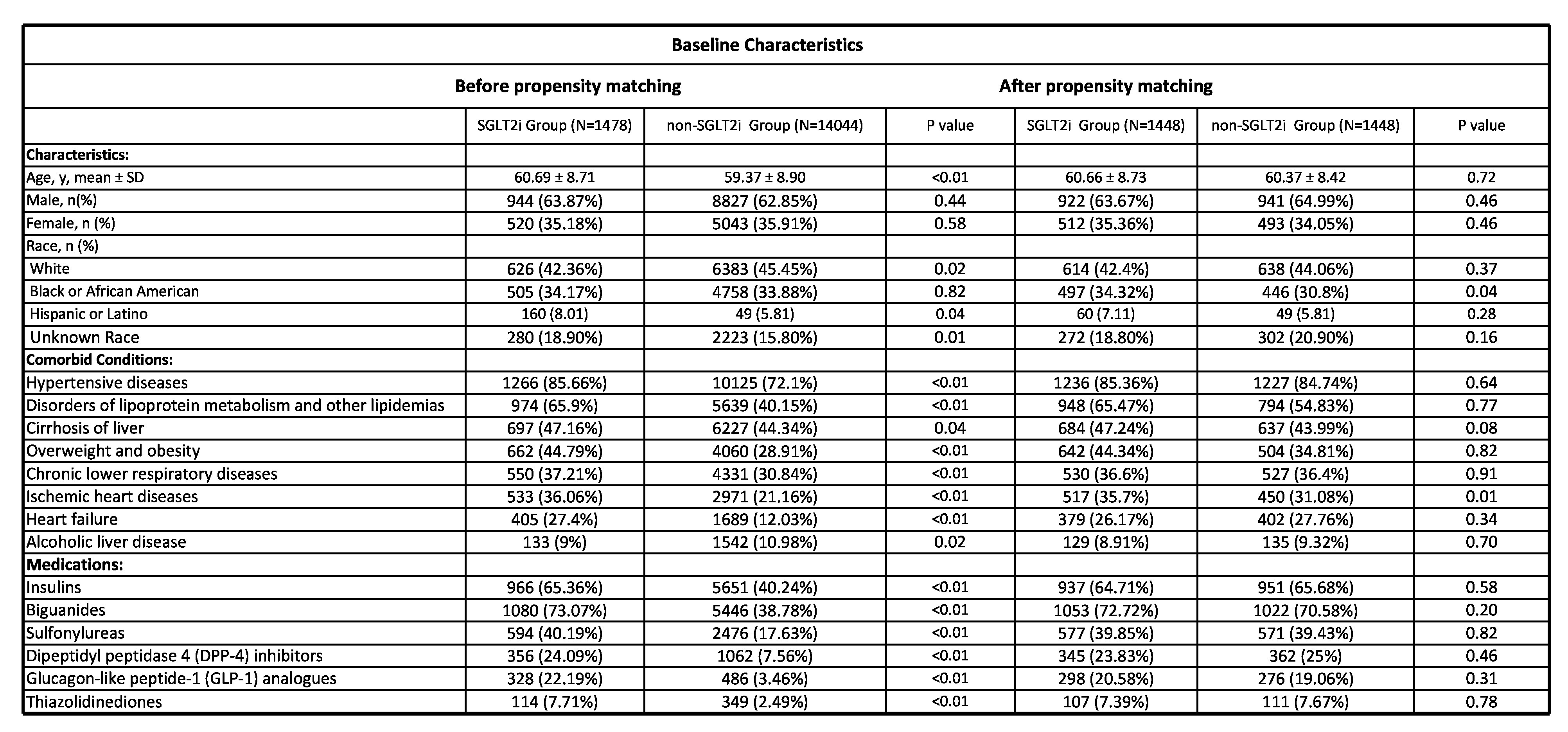
We identified 1,478 patients in the SGLT2i group and 14,044 patients in the non-SGLT2i group. A total of 1,448 patients were included after propensity matching from both groups (Figure 1). Table 1 contains the baseline demographics, comorbid conditions, and baseline medications before and after propensity matching. The incidence of HCC was 6.63% in the SGLT2i group vs. 10.42% in the non-SGLT2i group (RR = 0.64, 95% CI (0.49-0.81), P<0.01). This association remained significant regardless of age, gender, ethnicity, diabetes, hypertension, cirrhosis, and background diabetic medications (metformin, sulfonylurea, glitazone, DPP4i, alpha-glucosidase inhibitors, glucagon-like peptide 1 receptor agonists, and insulin).
Conclusion:
Based on a large global retrospective study of patients with co-existing CHC and T2DM, SGLT2i use was associated with a significantly lower risk of HCC. However, confirmation through future large-scale prospective studies is warranted.

Number: Sa1615
SGLT2I REDUCES THE RISK OF DEVELOPING HCC IN PATIENTS WITH CO-EXISTING HEPATITIS C INFECTION AND TYPE 2 DIABETES: A GLOBAL MULTICENTER PROPENSITY-MATCHED STUDY
Society: AASLD
Track: Liver Diseases and Transplantation
Introduction:
Chronic Hepatitis C infection (CHC) and Diabetes Mellitus Type 2 (T2DM) are independent risk factors for the development of Hepatocellular Carcinoma (HCC). Preclinical studies suggest that Sodium-glucose cotransporter-2 receptor inhibitors (SGLT2i) regulate pathways implicated in HCC progression and may inhibit oncogenesis. However, clinical studies are lacking. Using a large research network, this study aimed to evaluate the effect of SGLT2i on the incidence of HCC in patients with concomitant T2DM and CHC.
Methods:
In this retrospective cohort study and time-to-event analysis, we utilized the TriNetX electronic health records network, encompassing over 120 million patients from January 1, 2005, to December 31, 2022. We included patients aged 18 years or older diagnosed with co-existing T2DM and patients with CHC (who had received either previous or current antiviral treatment). The patients with and without SGLT2i were matched by propensity score for age, gender, race, comorbidities, liver-related characteristics, BMI, and background medications. Patients with a prior history of HCC, liver transplant, diabetes mellitus type 1, and end-stage renal disease were excluded from the study. Using a Cox proportional hazards regression model, we evaluated the association between SGLT2i use and the incidence of HCC in both groups.
Results:
We identified 1,478 patients in the SGLT2i group and 14,044 patients in the non-SGLT2i group. A total of 1,448 patients were included after propensity matching from both groups (Figure 1). Table 1 contains the baseline demographics, comorbid conditions, and baseline medications before and after propensity matching. The incidence of HCC was 6.63% in the SGLT2i group vs. 10.42% in the non-SGLT2i group (RR = 0.64, 95% CI (0.49-0.81), P<0.01). This association remained significant regardless of age, gender, ethnicity, diabetes, hypertension, cirrhosis, and background diabetic medications (metformin, sulfonylurea, glitazone, DPP4i, alpha-glucosidase inhibitors, glucagon-like peptide 1 receptor agonists, and insulin).
Conclusion:
Based on a large global retrospective study of patients with co-existing CHC and T2DM, SGLT2i use was associated with a significantly lower risk of HCC. However, confirmation through future large-scale prospective studies is warranted.